Protocol Application Process- Getting Started With a New or Existing Protocol
Welcome to the IRB Guidance page
This page is designed to help researchers navigate the Institutional Review Board (IRB) process for research involving human participants. Whether you're a student, faculty member, staff researcher or external collaborator, understanding your responsibilities is key to conducting ethical and complaint research.
See below details about research including human subject.
Step 0: Does your study require IRB review?
Before proceeding, determine whether your project qualifies as human subject research.
Take the IRB determination Survey
Disclaimer: The survey is a helpful starting point, but researchers should always consult with the IRB Office to confirm results.
IF your project does require IRB review, follow the 5 steps to IRB Success outlined below.
5 Steps to IRB Success
Approval/Review Timeline: The approval period depends upon the type of review (full, expedited, or exempt). Typically, most protocols are reviewed by the expedited method which can take 3-6 weeks, provided that the protocol packet is complete. Complete means that the protocol has all of its components and it has passed the administrative review* by the IRB office before it goes to a member of the IRB for ethical review.
*Administrative review can take 1-2 weeks.
*Please be advised that review timelines may be extended during the upcoming holiday period due to limited reviewer availability. We appreciate your understanding and encourage you to plan accordingly if you have time-sensitive research activities.
For student research guidance- Review the Faculty Information Sheet
Please ask questions at any stage of the process. The IRB Office is available and can help facilitate the review. You may reach us at 909-869-3713 (or 3771) or irb-office@cpp.edu See also the Institutional Review Board Staff Directory for additional contacts (IRB Chair and IRB Administrator)
STEP 1
- CITI is a leading provider of research education content. CITI training should be completed by PIs and all personnel listed on the protocol engaged in research
- CITI training is free for our campus community
- Registration Registration tutorial
- Register using your Bronco email and any password you choose.
- Select California State Polytechnic University, Pomona as the institution
- Provide your Bronco ID under "employee number"
- Selecting Courses
- select the group basic CPP Introduction to human subjects 101 Or
- Select CEIS Mandated Social Science Research (required for many social sciences)
- You must complete all courses with a passing score of 80% to receive a certificate of completion
- Certificate of completion should be provided to your Principle Investigator, Faculty Advisor or Professor
- CITI training is valid for five years, at which point, a renewal training must be completed
- Should you have any questions, contact the Office of Research Compliance irb-office@cpp.edu
- You may take any other modules since some professors and courses may have other requirements, e.g., some in CEIS require 80% for each module ( different than learner course (comprise of 6-10 modules).
- A transcript (completion report) is generated when you complete the required group of modules, which is evidence of training to the IRB or for a classroom activity. Five (5) years is the length of time for which the human subjects research training is valid. This is subject to change should there be changes in regulations, campus policies, or research methodology
- CITI Program issues course "Completion Certificates" (CCs) that learners may print or share, under the records link in the CITI site.
STEP 2
Request authentication. If this is your first time submitting an IRB protocol in Cayuse please email the following information (listed below) to irb-office@cpp.edu to be added into the system, additionally send a screenshot of your error message ( when attempting to log in https://cpp.cayuse424.com error message will read "We're sorry. This account has been disabled on the Cayuse424 system"):
- Highest degree attained (Ph.D., MS, MA, BS, BA, associates, high school, etc., as appropriate)
- First and last name
- Bronco ID (the nine-digit number, which will eventually be used to link with CITI training data)
- Bronco email (e.g., billybronco@cpp.edu)
- Status (faculty, staff, student, unaffiliated, etc., as appropriate)
- CPP College and Department
Authentication request are processed within 48-72 hours after emailing our office.
STEP 3
Train yourself to use Cayuse IRB.
- Review the Cayuse tutorial “Submitting a New Protocol” to learn how to navigate, complete, and submit an IRB protocol.
STEP 4
To submit an IRB protocol, enter the Cayuse IRB site using your Bronco Credentials (from #2 above) and start a "new study".
-
For the Cayuse IRB site to start or continue with an existing protocol, use https://cpp.cayuse424.com/
STEP 5
IRB protocol upkeep. To revise, amend (modify), renew, report an adverse event, or close your IRB protocol, follow as below:
Revise: After your protocol has been submitted to the IRB office, it will most often be returned to the PIs with comments. Learn how to navigate through revisions. 
Amend (modification): After IRB approval, when the PI(s) would like to change things to the approved protocol, they must submit an Amendment Submission.
Renew: When the protocol is close to the expiration date, the PIs will receive notifications. If they wish to renew the approval period for an additional year (the usual amount of time), a Renewal Submission must be submitted before the expiration date (earlier is better!).
Adverse Event (Incident): Sometimes things in research just don't go as planned. Researchers may encounter "adverse events" and unanticipated problems. Taken from OHRP, an adverse event is any untoward or unfavorable medical occurrence in a human subject, including any abnormal sign, symptom or disease temporally associated with the subject's participation in the research, whether or not considered related to the subject's participation in the research. Adverse events encompass both physical and psychological harms. Adverse events must be reported to the IRB within 48 hrs. through the Cayuse system.
Unanticipated problems include any incident, experience, or outcome that meets all of the following criteria:
- Unexpected (in terms of nature, severity, or frequency) given a) the research procedures that are described in the protocol-related documents, such as the IRB-approved research protocol and informed consent document; and b) the characteristics of the subject population being studied;
- Related or possibly related to participation in the research (possibly related means there is a reasonable possibility that the incident, experience, or outcome may have been caused by the procedures involved in the research); and
- Suggests that the research places subjects or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized.
Potential examples that relate to the typical kinds of research conducted at Cal Poly Pomona include (but are not limited to): an accident on the treadmill, a loss of datasheets with personally identifiable information, an emotional breakdown during a psychological interview, sickness following a food taste study, or a parent questioning the assent process of her child in an education project. And neither are all adverse events directed at the subject; for example, a vial of blood that breaks poses a bio-hazard to others. As a PI (principal investigator), one needs to think about the risks (the what-ifs) associated with the proposed research when writing the protocol; however, it is recognized that not everything is predictable.
According to federal regulation, it is the PI's responsibility to report adverse events of any kind to the IRB. Reporting adverse events promptly will enable the IRB and university research community to respond appropriately, to help the researchers continue their work, and to protect human volunteers in research investigations from avoidable harms. The criteria of how unexpected, how related, and how serious the event was will be evaluated and may necessitate a fresh look at the risk/benefit ratio and possibly a revision to the informed consent process.
Closure: If the data collection portion of the study is completed, a Closure Submission is submitted.
Getting started with a new/or existing protocol
A general, all-purpose, protocol:
General Sample Protocol 2015 (DOC.)- Investigators may use this sample protocol as a general guide to answer the questions on the protocol form. Note that every protocol is different and the format in which this protocol was written may not apply to every study but it could still be helpful.
- The most common reason for the delay in the revision phase is researchers’ delays in sending requested revisions and addressing the reviewer's comments. Protocols and revisions are reviewed in the order in which they are received, so every day counts.
If the activity is RESEARCH and involves HUMAN SUBJECTS, an IRB application is required for review and approval
*A few Cal Poly Pomona Graduate Programs require publication through Bronco Scholar, which then meets the requirements to submit an IRB application.
Human Subject: A living individual about whom an investigator (whether professional or student) conducting
research (1) obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens, or (2) obtains, uses analyzes, or generates identifiable private information or identifiable biospecimens.
Living individual: The specimen(s)/data/information must be collected from live subjects. Cadavers, autopsy specimens or specimens/information from subjects now deceased is not human subjects.
“About whom”: A human subject research project requires the data received from the living individual to be about the person.
Research: a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalize knowledge
Systematic Investigation: an activity that involves a prospective plan that incorporates data collection, either quantitative or qualitative, and data analysis to answer a question.
Generalizable Knowledge: designed to draw general conclusions, inform policy, or generalize findings beyond a single individual or an internal program (e.g., publications or presentations). However, research results do not have to be published or presented to qualify the experiment or data gathering as research. The intent to contribute to "generalize (scholarly) knowledge" makes an experiment or data collection research, regardless of publication.
Intervention: Includes both physical procedures by which data are gathered (for example, venipuncture) and manipulations of the subject or the subject's environment that are performed for research purposes.
Interaction: Includes communication or interpersonal contact between investigator and subject.
Private information: Includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a medical record). Identifiable private information is private information for which the identity of the subject is or may readily be ascertained by the investigator or associated with the information. An identifiable biospecimen is a biospecimen for which the identity of the subject is or may readily be ascertained by the investigator or associated with the biospecimens.
Identifying Human Research Studies
Certain studies may have the characteristics of human subjects research but may not meet the regulatory definition. Studies which meet the definition require IRB review. There are three categories to consider:
- Studies that are human subjects research
- Studies that may be considered human subjects research (gray area)
- Studies that do not qualify as human subjects research
Any investigator who is unsure of whether his/her proposal constitutes “human subjects research” should contact the IRB office irb-office@cpp.edu . The IRB Chair, compliance associate and/or designee will determine if the study is human subjects research. Federal regulation do not recommend investigators to make this determination themselves.
Studies that ARE Human Subjects Research
- Studies that utilize test subjects for new devices, products, drugs, or materials.
- Studies that collect data through intervention or interaction with individuals. Examples of this type of research include Internet surveys about alcohol consumption, studies that involve deception, research involving risky behaviors or attitudes, and open-ended interviews with minors that contribute to generalize knowledge.
- Studies using private information that can be readily identified with individuals, even if the information was not collected specifically for the study in question.
- Studies that use bodily materials such as cells, blood, urine, tissues, organs, hair, or nail clippings, even if the investigator did not collect these materials for the study.
- Studies that reproduce generalize knowledge about categories or classes of subjects from individually identifiable information.
- Studies that use human beings to evaluate environmental alterations, for example, weatherization options or habitat modification to their living or working space or test chamber.
Studies that ARE NOT human subjects research
Studies that fit any of the categories below do not need an IRB review
- Data Collection ( of secondary data with no identifiers, and dependant on method of collection/for what intended purposes)
- Service Surveys
- Course-related Activities
- Biography or Oral History Research
- Quality Improvement
- Case Histories
- Publicly Available Data
- Coded Private Information or Biological Specimens
A pilot study is a preliminary investigation of the feasibility of a study, usually done on a small scale (approximately 10 or fewer subjects) and exploratory in nature. It is designed to help the investigator refine data collection procedures and instruments or prepare a better, more precise research design. Such a pilot would not contribute to generalize knowledge and therefore is not considered research and does not require IRB review. Data collected from a pilot study cannot be used as research data.
If you are recruiting volunteers through a faculty, department, organization, or agency, or if you are conducting research in the classroom, or at a business, organization, or agency:
- Letter of permission (by the designated approver) to recruit and/or conduct research (on official letterhead) must be submitted with your application.
-
Informed consent forms (ICFs):
Child Assent forms:
Child assent form 7-10 years old
Child assent form 11-14 years old
Child assent form 15-17 years old
Anonymous* and non-intrusive study:
Anonymous and non-intrusive study
Confidential* study:
SPANISH Informed Consent Form ( appropriate revisions must be made by the investigator)
Non-Disclosure Agreement- For Transcribers
NDA For the CPP IRB, a non-disclosure agreement (NDA) ensures that discussions pertaining to the research are kept confidential.
Site- Authorization
Site authorization is a written permission from an organization to conduct research (i.e., school, private business, or medical office) at that site. This site-authorization should be included in your CP protocol application. This authorization ensures you have proper permissions and support to collect data. If the site is also your employer, ensure you provide the IRB with sufficient information about the accessing internal data.
Other relevant resources about ICFs:
Example statements for CPP graduate students to use in their consenting forms, meant to avoid undue influence and coercion when recruiting subjects, is available here in the document "Undue Influence and Coercion in Consenting".
Experimental subject's bill of rights for medical research This document is often used for biomedical/clinical procedures.
* Anonymous versus Confidential Often the difference between the terms anonymous and confidential is not well understood when applied to research studies involving human participants. Yet, these concepts are very important for the protection of the subjects, and they need to be considered by researchers when designing a study and writing the ICF. During protocol review, the level of protection described will be evaluated by the IRB.
OHRP FAQs on informed consent This website from the federal Office for Human Research Protections (OHRP) offers information about the consent and assent processes.
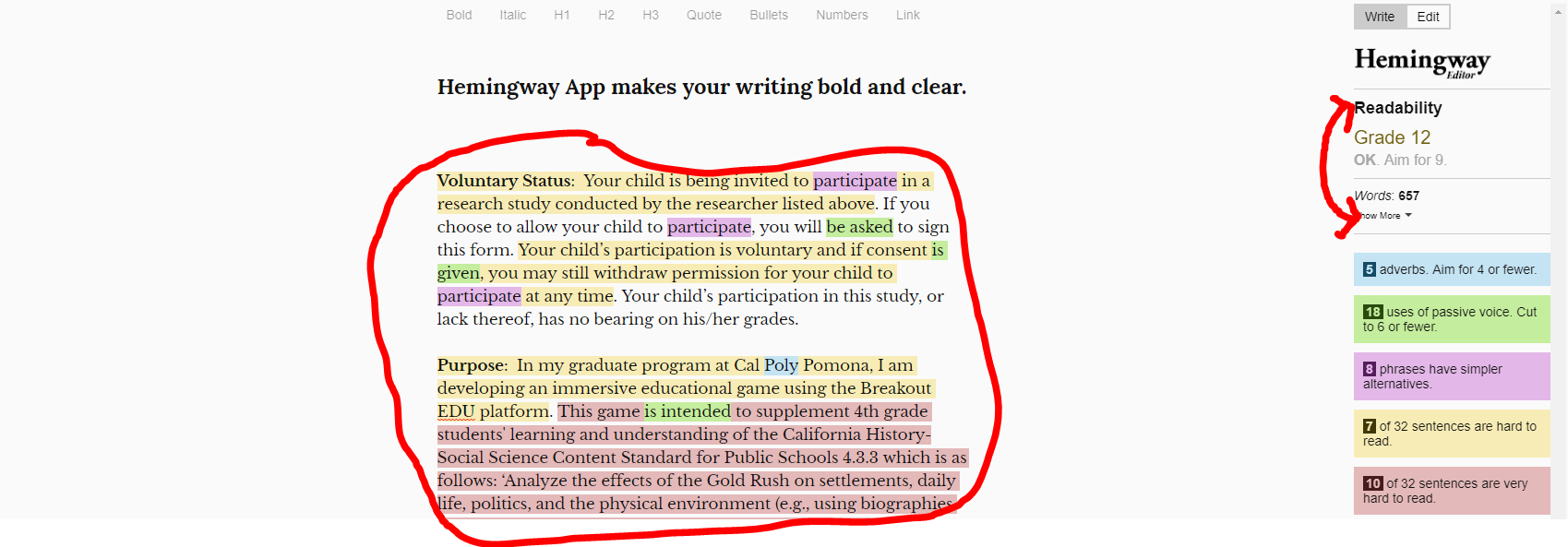
Evaluating the comprehension level or readability factor of a document like the ICF can be done with tools like these. IRB protocol reviewers may ask you as the PI to provide the result or score from them.
- http://www.hemingwayapp.com/
- Remove all text on the page and insert yours, your grade score will display for ICF must be 6th or 7th grade, assent forms 5th grade
Researchers must attach ALL documents and scripts seen, heard, signed, or answered by potential or consenting participants, which may include:
- Recruiting script email or recruiting flyers/letters/ads
- Informed Consent Document(s) or Implied Consent Document(s) for online surveys
-
Final survey
-
Interview questions
-
Focus Group questions
-
Debriefing Document(s) for research with deception
-
Photo Releases (if using photographs of participants)
-
Video Releases (if video recording participants)
-
Recruiting script/text or recruiting flyers/letters/ads
Qualtrics
Questions about research data?
Review this publication on "Access to and Retention of Research Data: Rights and Responsibilities" Council on Governmental Relations, 2012
Questions about using copyrighted materials?
The CSU Office of General Counsel has a manual on the Fundamentals of Copyright and Fair Use. See:
Fundamentals of Copyright and Fair UseFAQ's
To submit a new protocol, navigate to the "Protocol Application " tab. The protocol is an electronic document, within the Cayuse IRB software. After authorizing/authenticating users to gain access to Cayuse IRB, you may complete the protocol and submit it for review by the IRB.
On the other hand, if you wish to develop your IRB protocol using a Word document protocol application as a template, it is available on the "Protocol Application" page under section 4 “To submit an IRB protocol”. This is useful in the classroom and for practicing. To officially submit the protocol, the content will need to be cut-and-pasted into the protocol within the Cayuse software.
When considering submitting a protocol for IRB review and approval, there are a few questions you should keep in mind. The Compliance Office will evaluate the need for submission based on the following question along with other determining factors;
- Does my activity involve research?
- Does the research involve human subjects?
- Is the human subject research exempt from submission or will be be categorized as exempt from IRB oversight?
Please refer to the following definitions to determine whether your research study will require IRB review and approval. There is a lot of "grey" when interpreting the federal regulations
For the purposes of the IRB, “human subject research” is defined in 45 CFR 46.102(e) of the Code of Federal Regulations TITLE 45 DHHS PART 46 PROTECTION OF HUMAN SUBJECTS.
Human subject - a living individual about whom an investigator (whether professional or student) conducting research obtains:
- Data through intervention or interaction with the individual, or
- Identifiable private information.
Intervention includes both physical procedures by which data are gathered (for example, conjuncture) and manipulations of the subject or the subject's environment that are performed for research purposes.
Interaction includes communication or interpersonal contact between investigator and subject. This includes survey and questionnaires, even if there is no direct contact between the investigator and subject.
Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, medical records or student records). Private information must be individually identifiable (i.e., the identity of the subject is or may readily be ascertained by the investigator or associated with the information) in order for obtaining the information to constitute research involving human subjects.
Not all human subject research falls under Federal Policy, and there may be differences in the types of review required based on the potential risk to the subjects. However, only an IRB can determine if a given research project falls under Federal Policy or which aspects of the policy apply. Under Federal Policy, an IRB can decide on policy that is more restrictive than Federal Policy, though never more lenient.
Please read Section 14 (Special topics Research at Cal Poly Pomona by Unaffiliated Investigators in the IRB Policy & Procedures manual. If you wish to submit an IRB protocol to CPP as an unaffiliated investigator, please email the IRB office (irb-office@cpp.edu) the following information/attachments:
- Your name, title, home institution
- Summary of your study pertaining to CPP
- Name of PI
- Name of co-PI(s) (must be CPP affiliated)
- All study documents that were approved by your home institution and IRB approval letter/memo (final IRB protocol, consent forms, recruitment forms, authorizations, data collection documents, IRB approval memos, etc.)
Submission of the above does not indicate approval from CPP INSTITUTIONAL REVIEW BOARD.
The approval period depends upon the type of review (full, expedited, or exempt). Typically, most protocols are reviewed by the expedited method which can take 3-6 weeks, provided that the protocol packet is complete. Complete means that the protocol has all of its components and it has passed the administrative review* by the IRB office before it goes to a member of the IRB for ethical review.
*Administrative review can take 1-2 weeks.
When the review process has been completed, you will receive an email informing you that your protocol has been approved. A copy is also contained in the "Letters" tab of the Cayuse submissions page. It serves as the official notification/letter that the ethical review has been done and your research study has been approved for conduct.
Per federal regulations, protocols can be approved for conduct for up to one year before the renewal process would occur, unless it is exempted from IRB oversight, in which case the CPP Institutional Review Board will determine the renewal dates.
*The determination is made during the protocol review.
In the case of most protocols, all data collection documents should be attached to the protocol that is being submitted for approval. If for some reason a document is not finalized, explain this in the “Data Collection” section of your initial protocol, including a description of your intended methodology. Once the documents are finalized, submit them to the IRB office through the Cayuse IRB process.
A protocol cannot be approved for conduct without a review of the data collection documents. A notable exception is when the objective of the protocol is to develop data collection processes such as reliable and validated surveys.
Any change to a document that has been previously approved by the IRB will require an amendment form for review and approval.
Should there be any changes to your research plan as described in the approved and current protocol, you will need to submit an amendment, also called a modification. See below questions, " How to submit a change to an IRB approved protocol".
Examples of changes include, but are not limited to:
- A change of study design, methodology, or recruitment methods
- Changes to any data collection documents, including surveys and questionnaires
- Changes to consent documents
- Changes to the population proposed in the approved protocol
- Changes in funding
- Addition/Deletion of investigators (PIs), co-PIs, and research assistants
- Change of Project Title
- Addition/Deletion of research performance sites
No.
After the expiration date, you may no longer continue to collect data. According to federal regulation investigators must not engage in research with with subjects after a protocol's expiration date . However you may continue to work on analysis of the data. This is because the interaction with subjects/participants has concluded.
The Cayuse IRB protocol software sends a renewal reminder as a courtesy to you before the expiration of the approval. If you have received a notice and your project is not complete, contact the IRB office to inquire about options
*PIs are responsible for knowing the expiration status of their protocol.
If your protocol's expiration date has passed, then you can no longer renew the protocol and you cannot conduct any more research that engages the subjects. This is according to federal regulations.
You must submit a protocol again. It is a de novo process with a new protocol number, IRB member review, submission of all documents, etc. do not ignore those messages if you plan to continue working with human subjects.
Per federal regulation, it is the PI's responsibility to report adverse events of any kind to the IRB. Reporting adverse events promptly will enable the IRB and university research community to respond appropriately, to help the researchers continue their work, and to protect human subjects in research investigations from avoidable harms.
To submit an adverse event report through Cayuse. Use the "+ new submission" button and choose the option "Incident".
Follow this link for instructions from the CITI group on how to obtain your CITI training report and determine whether your research ethics training needs to be renewed/updated.
You can test the consent form by inserting only the text of the document into a readability calculator, like: