IRB

Is IRB Approval Required for Student Research Projects that Involve Human Subjects?
The answer is "YES" in most cases. Student research, such as class projects and poster presentations have the same requirements as any other studies that involve human participation. Unless it is a student class assignment which will not be published, then it does not require an IRB application, approval, or oversight.
Some data which has previously been collected may be considered Non-Human Subject Research if it is unidentifiable/de-identified or coded private information which cannot readily ascertain the identities of the individuals to whom the data or samples belong to. In these cases, an IRB application, approval, or oversight may not be required. Contact the IRB office with details of your proposed research to confirm: irb-office@cpp.edu
Does Your Project Require an IRB Application?
Your project may require an IRB application depending on a few factors:
- Minimal Risk - The probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.
- Full Review - Typically, studies which present more than minimal risk to subjects or involve vulnerable participants* (children, pregnant women, prisoners) must be evaluated by the entire committee, during their monthly meeting to collectively review the proposed study. These studies are assigned a check-in date or expiration date.
- *Studies involving prisoners, persons of undocumented status, research on illegal activities, incarcerated youth, among others.
- Expedited Review - The process of reviewing a protocol by (usually) one or two members of the IRB because the study has potentially minimal risk to the human subjects.
- Exempt Review - The process of determining, by the IRB, that a protocol is not subject to either expedited review or full review, these are studies normally collect data anonymously or if they are identifiable surveys (or interviews) the identity of the subjects cannot be readily ascertained.

- Confirm that your project is "Human Subject Research"
- Verify with your faculty advisor OR you can always contact the IRB office if you need help assessing this.
- Email the IRB staff for drop-in hours, Monday and Wednesday at 10:00 - 11:00 am.
- For more guidance see the IRB’s Policies and Guidelines.
- Verify with your faculty advisor OR you can always contact the IRB office if you need help assessing this.
- Complete your required Human Subject Research Education training
- See Step 1 in Getting Started with a New or Existing Protocol
- Create a Cayuse IRB account. Once created, you will be notified and then have access to the Cayuse IRB platform.
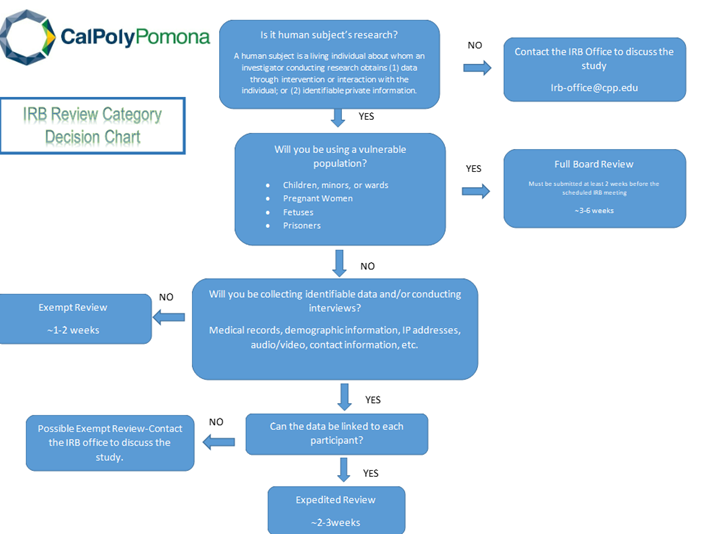
- Select the most appropriate IRB review category (exempt, expedited or full board) based on your study's risk level.
- Complete your Cayuse IRB application: Include surveys, informed consent forms, external approvals and ensure your faculty advisor has reviewed and approved your proposed study.
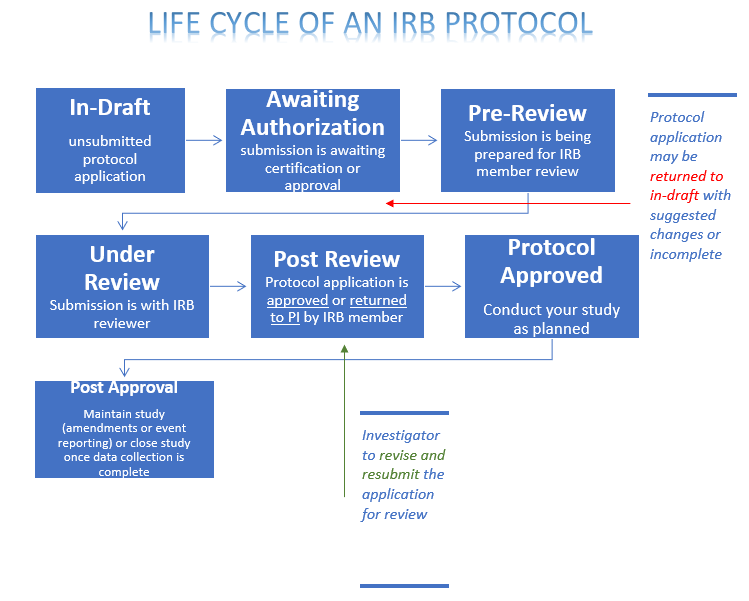
- An IRB protocol application has 10 sections. You may already have most of the required information in the research proposal you and your faculty advisor have developed.
- The image below shows the step-by step process your IRB application goes through.

IRB Definitions
- Research - A systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. Activities which meet this definition constitute research for purposes of these guidelines, whether or not they are conducted or supported under a program which is considered research for other purposes. For example, some demonstration and service programs may include research activities. For the purposes of these guidelines, a “systematic investigation” is an activity that involves a prospective research plan which incorporates data collection, both quantitative and/or qualitative, and data analysis to answer a research question. Investigations designed to develop or contribute to generalizable knowledge are those designed to draw general conclusions (i.e., knowledge gained from a study may be applied to populations outside of the specific study population), inform policy, or generalize findings.
- Minimal Risk – The probability and magnitude of physical or psychological harm that is normally encountered in the daily lives, or in the routine medical, dental, or psychological examination of healthy persons.
- Intervention - Includes physical procedures by which information (data) are gathered and manipulations of the subject or the subject’s environment are performed for research purposes.
- Interaction - Includes communication or interpersonal contact between investigator and subject.
- Identifiable Private Information - Private information for which the identity of the subject is or may readily be ascertained by the investigator or associated with the information.
- Confidential - Means that the researcher knows who the respondent is but is keeping such information concealed.
- Anonymous - Can involve interviewing without ever collecting identifying information.
-
Human Subject - Means a living individual about whom an investigator (whether professional or student) conducting research:
-
Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or
- Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.
-
IRB Resources

CPP IRB Website

Links to Resources
Cayuse Tutorial - Navigating the IRB Site

IRB Guidelines & Procedures


Tips From Diana
Fellow Researcher and CPP Alumna
- Ensure both, you and your faculty advisor have discussed and agreed to submit your research to the IRB for review and approval. Going into it you should have a general research proposal outline that you will reference during the application process.
- PLAN IT OUT! IRB application is time consuming. The clearer and more detailed you are, the higher the probability of getting approval on your 1st try.
- It’s okay to feel overwhelmed! This is a big step into your research endeavor, and you will come out a stronger researcher in the end. Communicate with your faculty advisor and reach out for help if needed!
- Review your application before submission! Make your faculty advisor approves of your application prior to submission. Once your faculty agrees with your writing, and no additional changes need to be made hit submit for IRB pre-review!
- *Make sure to write out it out in future tense in your IRB application*
- Turnaround time can vary (2-4 weeks at minimum) so plan accordingly.
- Once you receive and email approval from Cayuse (it will state your research start date) check in with your faculty mentor to begin data collection!
FFTs
Along with IRB or anything involving a new task, try going into it with an FFT mindset:
“...FFTs (effing first times): how hard it is to be new at things – from small things to global pandemics. When we have no relevant experience or expertise, the vulnerability, uncertainty, and fear of these firsts can be overwhelming. Yet, showing up and pushing ourselves past the awkward, learner stage is how we get braver.”
- Brené Brown
FFT will provide you with ease and help guide you through your CPP journey. Best of luck with research!